The Si2H radical supported by two N-heterocyclic carbenes†
Abstract
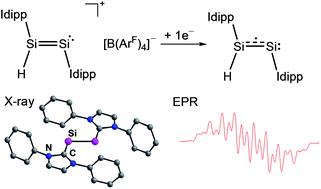
Cyclic voltammetric studies of the hydridodisilicon(0,II) borate [(Idipp)(H)SiII![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si0(Idipp)][B(ArF)4] (1H[B(ArF)4], Idipp = C[N(C6H3-2,6-iPr2)CH]2, ArF = C6H3-3,5-(CF3)2) reveal a reversible one-electron reduction at a low redox potential (E1/2 = −2.15 V vs. Fc+/Fc). Chemical reduction of 1H[B(ArF)4] with KC8 affords selectively the green, room-temperature stable mixed-valent disilicon(0,I) hydride Si2(H)(Idipp)2 (1H), in which the highly reactive Si2H molecule is trapped between two N-heterocyclic carbenes (NHCs). The molecular and electronic structure of 1H was investigated by a combination of experimental and theoretical methods and reveals the presence of a π-type radical featuring a terminal bonded H atom at a flattened trigonal pyramidal coordinated Si center, that is connected via a Si–Si bond to a bent two-coordinated Si center carrying a lone pair of electrons. The unpaired electron occupies the Si
Si0(Idipp)][B(ArF)4] (1H[B(ArF)4], Idipp = C[N(C6H3-2,6-iPr2)CH]2, ArF = C6H3-3,5-(CF3)2) reveal a reversible one-electron reduction at a low redox potential (E1/2 = −2.15 V vs. Fc+/Fc). Chemical reduction of 1H[B(ArF)4] with KC8 affords selectively the green, room-temperature stable mixed-valent disilicon(0,I) hydride Si2(H)(Idipp)2 (1H), in which the highly reactive Si2H molecule is trapped between two N-heterocyclic carbenes (NHCs). The molecular and electronic structure of 1H was investigated by a combination of experimental and theoretical methods and reveals the presence of a π-type radical featuring a terminal bonded H atom at a flattened trigonal pyramidal coordinated Si center, that is connected via a Si–Si bond to a bent two-coordinated Si center carrying a lone pair of electrons. The unpaired electron occupies the Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si π* orbital leading to a formal Si–Si bond order of 1.5. Extensive delocalization of the spin density occurs via conjugation with the coplanar arranged NHC rings with the higher spin density lying on the site of the two-coordinated silicon atom.
Si π* orbital leading to a formal Si–Si bond order of 1.5. Extensive delocalization of the spin density occurs via conjugation with the coplanar arranged NHC rings with the higher spin density lying on the site of the two-coordinated silicon atom.

 Please wait while we load your content...
Please wait while we load your content...