β-Glutamine-mediated self-association of transmembrane β-peptides within lipid bilayers†
Abstract
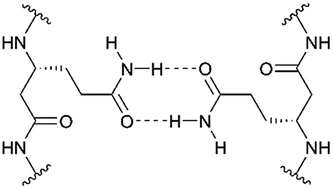
Transmembrane β-peptide helices and their association in lipid membranes are still widely unexplored. We designed and synthesized transmembrane β-peptides harboring different numbers of D-β3-glutamine residues (hGln) by solid phase peptide synthesis. By means of circular dichroism spectroscopic measurements, the secondary structure of the β-peptides reconstituted into unilamellar vesicles was determined to be similar to a right-handed 314-helix. Fluorescence spectroscopy using D-β3-tryptophan residues strongly suggested a transmembrane orientation. Two or three hGln served as recognition units between the helices to allow helix–helix assembly driven by hydrogen bond formation. The association state of the transmembrane β-peptides as a function of the number of hGln residues was investigated by fluorescence resonance energy transfer (FRET). Therefore, two fluorescence probes (NBD, TAMRA) were covalently attached to the side chains of the transmembrane β-peptide helices. The results clearly demonstrate that only β-peptides with hGln as recognition units assemble into oligomers, presumably trimers. Temperature dependent FRET experiments further show that the strength of the helix–helix association is a function of the number of hGln residues in the helix.

 Please wait while we load your content...
Please wait while we load your content...