Non-bonding 1,5-S⋯O interactions govern chemo- and enantioselectivity in isothiourea-catalyzed annulations of benzazoles†
Abstract
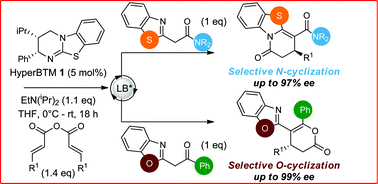
Isothiourea-catalyzed annulations between 2-acyl benzazoles and α,β-unsaturated acyl ammonium intermediates are selectively tuned to form either lactam or lactone heterocycles in good yields (up to 95%) and high ee (up to 99%) using benzothiazole or benzoxazole derivatives, respectively. Computation gives insight into the significant role of two 1,5-S⋯O interactions in controlling the structural preorganization and chemoselectivity observed within the lactam synthesis with benzothiazoles as nucleophiles. When using benzazoles the absence of a second stabilizing non-bonding 1,5-S⋯O interaction leads to a dominant C–H⋯O interaction in determining structural preorganization and lactone formation.

 Please wait while we load your content...
Please wait while we load your content...