A supramolecularly tunable chiral diphosphine ligand: application to Rh and Ir-catalyzed enantioselective hydrogenation†
Abstract
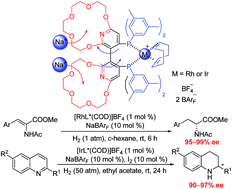
A supramolecularly tunable chiral bisphosphine ligand bearing two pyridyl-containing crown ethers, (−) or (+)-Xyl-P16C6-Phos, was fabricated and utilized in the Rh-catalyzed asymmetric hydrogenation of α-dehydroamino acid esters and Ir-catalyzed asymmetric hydrogenation of quinolines in high yields with excellent enantioselectivities (90–99% ee). Up to a 22% enhancement in enantioselectivity was achieved by the addition of certain amounts of alkali ions (Li+, Na+ or K+), which could be selectively recognized and effectively complexed by the crown ethers on the chiral Xyl-P16C6-Phos.

 Please wait while we load your content...
Please wait while we load your content...