Post-synthetic modification of a macrocyclic receptor via regioselective imidazolium ring-opening†
Abstract
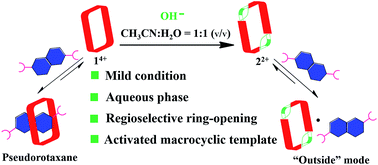
A facile post-synthetic modification of a tetracationic tetraimidazolium macrocycle, 14+ (i.e., the “Texas-sized” molecular box (cyclo[2](2,6-di(1H-imidazol-1-yl)pyridine)[2](1,4-dimethylenebenzene)), is described. Under mild basic conditions, ring-opening of the imidazolium moieties occurs. This results in two new isomeric dicationic macrocycles. This simple yet efficient modification serves to alter the size of the molecular cavity, the charge of the macromolecular receptor, and the manner whereby it interacts with dianionic guest molecules. The isomeric mixture of imidazolium ring opened macrocycles can be synthesized in relatively high overall yield (86–93%). The reaction shows regioselectivity and the ratio of major to minor (i.e., trans : cis ring-opened products) was determined to be ca. 3 : 1 via1H NMR spectroscopy. The major isomer, trans-cyclo[2]((Z)-N-(2-((6-(1H-imidazol-1-yl)pyridin-2-yl)amino)vinyl)formamide)[2](1,4-bismethylbenzene) hexafluorophosphate (22+·2PF6−), was isolated in its pure form in 42% yield via recrystallization. The molecular recognition properties of 22+ were investigated using a series of dianionic guests (i.e., 2,6-naphthalenedicarboxylate (4), 2,6-naphthalenedisulfonate (5), and 1,5-naphthalenedisulfonate (6)) whose binding interactions with 14+ have been previously reported. This allowed us to evaluate how imidazolium ring-opening affects the inherent host/guest interactions of 14+. On the basis of solution spectroscopic studies (e.g., 1H NMR, 1H–1H COSY NMR, DOSY NMR, and NOESY NMR), in tandem with mass spectrometric analyses (ESI-MS) and single-crystal X-ray diffraction studies, we conclude that opening up the macrocyclic structure (i.e., converting 14+ to 22+) leads to considerable changes in the recognition behavior, with so-called outside binding or weak ion pair interactions, rather than pseudorotaxane formation, being favored both in solution and the solid-state. We postulate that methodologies such as those described herein could provide a means to control the molecular interactions of both free-standing macrocycles and those used to construct mechanically-interlocked molecules. Indeed, the application of hydroxide anion under the present conditions not only serves to effect the ring-opening of 14+, but also pseudorotaxane structures, such as, e.g., [14+·4] or [14+·5] derived there from.
- This article is part of the themed collection: ISACS18: Challenges in Organic Materials and Supramolecular Chemistry

 Please wait while we load your content...
Please wait while we load your content...