On the prevalence of bridged macrocyclic pyrroloindolines formed in regiodivergent alkylations of tryptophan†
Abstract
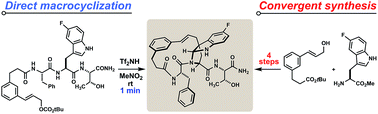
A Friedel–Crafts alkylation is described that efficiently transforms tryptophan-containing peptides into macrocycles of varying ring connectivity. Factors are surveyed that influence the distribution of regioisomers, with a focus on indole C3-alkylations leading to bridged endo-pyrroloindolines. We probe the stability and stereochemistry of these pyrroloindolines, study their rearrangement to C2-linked indolic macrocycles, and demonstrate a scalable, stereoselective synthesis of this compound class. Placing the macrocyclization in sequence with further template-initiated annulation leads to extraordinary polycyclic products and further demonstrates the potential for this chemistry to drive novel peptidomimetic lead discovery programs.

 Please wait while we load your content...
Please wait while we load your content...