Catalytic discrimination between formyl groups in regio- and stereoselective intramolecular cross-aldol reactions†
Abstract
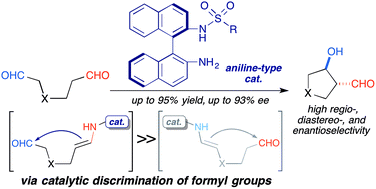
Catalytic discrimination between inequivalent formyl groups was achieved using an aniline-type acid–base catalyst for the regio-, diastereo-, and enantioselective intramolecular cross-aldol reactions of enolizable dials. Although L-proline gave a mixture of the regio- and stereoisomeric products in the presence of an N-containing 1,6-dial, the aniline-type catalyst afforded anti-3,4-disubstituted pyrrolidine in high regio-, and stereoselectivity beyond the background reaction, which led to the regioisomeric 2,3-disubstituted products. The mild reactivity of the aniline-type amine facilitated catalytic discrimination between the inequivalent formyl groups. Kinetic isotope effect studies and reductive amination experiments suggested that the regioselectivity was controlled under the enamine-forming steps.

 Please wait while we load your content...
Please wait while we load your content...