Determination of enantiomeric excess of carboxylates by fluorescent macrocyclic sensors†
Abstract
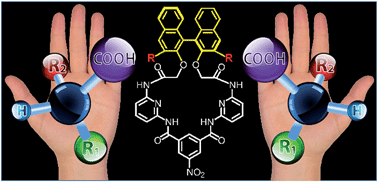
Chiral fluorescent chemosensors featuring macrocycles comprising BINOL auxiliary and an array of hydrogen bond donors were synthesized. To enhance fluorescence of the chemosensors, conjugated moieties were attached to the 3,3′-positions of the BINOL auxiliary. The resulting chemosensors recognize a number of carboxylates, namely, enantiomers of ibuprofen, ketoprofen, 2-phenylpropanoate, mandelate, and phenylalanine in a stereoselective fashion. Depending on the structure of the chemosensor, the presence of carboxylate yields fluorescence quenching or amplification. This information-rich signal can be used to determine the identity of the analyte including the sense of chirality. Quantitative experiments were performed aimed at analysis of enantiomeric excess of chiral carboxylates. The quantitative analysis of enantiomeric composition of ibuprofen, ketoprofen, and phenylalanine shows that the sensors correctly identify mixtures with varying enantiomeric excess and correctly predict the enantiomeric excess of unknown samples with error of prediction <1.6%.

 Please wait while we load your content...
Please wait while we load your content...