Calcium ions tune the zinc-sequestering properties and antimicrobial activity of human S100A12†
Abstract
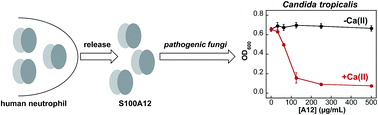
Human S100A12 is a host-defense protein expressed and released by neutrophils that contributes to innate immunity. Apo S100A12 is a 21 kDa antiparallel homodimer that harbors two Ca(II)-binding EF-hand domains per subunit and exhibits two His3Asp motifs for chelating transition metal ions at the homodimer interface. In this work, we present results from metal-binding studies and microbiology assays designed to ascertain whether Ca(II) ions modulate the Zn(II)-binding properties of S100A12 and further evaluate the antimicrobial properties of this protein. Our metal-depletion studies reveal that Ca(II) ions enhance the ability of S100A12 to sequester Zn(II) from microbial growth media. We report that human S100A12 has antifungal activity against Candida albicans, C. krusei, C. glabrata and C. tropicalis, all of which cause human disease. This antifungal activity is Ca(II)-dependent and requires the His3Asp metal-binding sites. We expand upon prior studies of the antibacterial activity of S100A12 and report Ca(II)-dependent and strain-selective behavior. S100A12 exhibits in vitro growth inhibitory activity against Listeria monocytogenes. In contrast, S100A12 has negligible effect on the growth of Escherichia coli K-12 and Pseudomonas aeruginosa PAO1. Loss of functional ZnuABC, a high-affinity Zn(II) import system, increases the susceptibility of E. coli and P. aeruginosa to S100A12, indicating that S100A12 deprives these mutant strains of Zn(II). To evaluate the Zn(II)-binding sites of S100A12 in solution, we present studies using Co(II) as a spectroscopic probe and chromophoric small-molecule chelators in Zn(II) competition titrations. We confirm that S100A12 binds Zn(II) with a 2 : 1 stoichiometry, and our data indicate sub-nanomolar affinity binding. Taken together, these data support a model whereby S100A12 uses Ca(II) ions to tune its Zn(II)-chelating properties and antimicrobial activity.


 Please wait while we load your content...
Please wait while we load your content...