Base-catalyzed synthesis of aryl amides from aryl azides and aldehydes†
Abstract
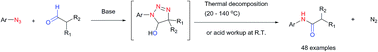
Aryl amides have been used as important compounds in pharmaceuticals, materials and in molecular catalysis. The methods reported to prepare aryl amides generally require very specific reagents, and the most popular carboxyl–amine coupling reactions demand stoichiometric activators. Herein, we report that aryl azides react with aldehydes under base-catalyzed conditions to yield aryl amides efficiently. Mechanistic investigations support the formation of triazoline intermediates via azide–enolate cycloaddition, which subsequently undergo rearrangement to give amides by either thermal decomposition (20–140 °C) or aqueous acid work-up at room temperature. The strategy does not require nucleophilic anilines and is especially efficient for highly electron-deficient aryl amides, including perfluoroaryl amides, which are otherwise challenging to synthesize.

 Please wait while we load your content...
Please wait while we load your content...