A significant change in selective adsorption behaviour for ethanol by flexibility control through the type of central metals in a metal–organic framework†
Abstract
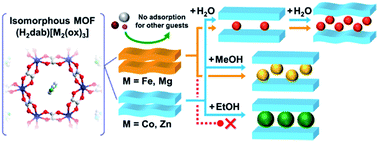
Closed–open structural transformations in flexible metal–organic frameworks (MOFs) are of interest for potential applications such as separation, because of their complete selectivity for the adsorption of specific guest molecules. Here, we report the control of the adsorption behaviour in a series of flexible MOFs, (H2dab)[M2(ox)3] (H2dab = 1,4-diammoniumbutane, M = Fe, Co, Ni, Zn, or Mg), having different central metals with analogous crystal structures. We found that a significant change in the selective adsorption behaviour for EtOH over MeCHO and MeCN is caused by the type of central metals, without changes in the crystal structures of all phases (except the Ni compound). A systematic study of adsorption measurements and structural analyses of the analogous MOFs reveals for the first time that the framework flexibility around the central metals of MOFs is truly related to the selective adsorption behaviour.


 Please wait while we load your content...
Please wait while we load your content...