Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal–organic framework cathode†
Abstract
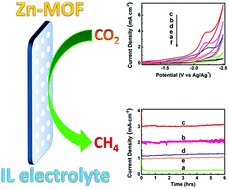
Highly efficient electrochemical reduction of CO2 to CH4 is of great importance, but is challenging. Herein, Zn–1,3,5-benzenetricarboxylic acid metal–organic frameworks (Zn–BTC MOFs) deposited on carbon paper (CP) were used as cathodes in electrochemical reduction of CO2 using ionic liquids (ILs) as the electrolytes, which was the first work on combination of a MOF electrode and an pure IL electrolyte in the electrochemical reduction of CO2. It was found that the efficiency of the reaction depended strongly on the morphology of the Zn-MOFs. Compared with the commonly used metal electrodes, the electrochemical reaction showed much higher selectivity to CH4 and current density, and the overpotentials for CH4 is much lower. The excellent combination of the MOF cathodes and ILs opens a way for reduction of CO2 to CH4 effectively.
- This article is part of the themed collections: Global Energy Challenges: Electrochemical Energy and Top 50 Articles of 2016: Energy and Catalysis

 Please wait while we load your content...
Please wait while we load your content...