Using the hydrogen and oxygen in water directly for hydrogenation reactions and glucose oxidation by photocatalysis†
Abstract
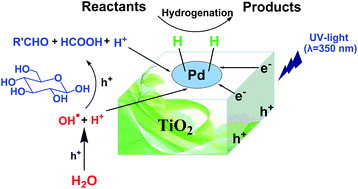
Direct utilization of the abundant hydrogen and oxygen in water for organic reactions is very attractive and challenging in chemistry. Herein, we report the first work on the utilization of the hydrogen in water for the hydrogenation of various organic compounds to form valuable chemicals and the oxygen for the oxidation of glucose, simultaneously by photocatalysis. It was discovered that various unsaturated compounds could be efficiently hydrogenated with high conversion and selectivity by the hydrogen from water splitting and glucose reforming over Pd/TiO2 under UV irradiation (350 nm). At the same time, glucose was oxidated by the hydroxyl radicals from water splitting and the holes caused by UV irradiation to form biomass-derived chemicals, such as arabinose, erythrose, formic acid, and hydroxyacetic acid. Thus, the hydrogen and oxygen were used ideally. This work presents a new and sustainable strategy for hydrogenation and biomass conversion by using the hydrogen and oxygen in water.

 Please wait while we load your content...
Please wait while we load your content...