A flexible iron(ii) complex in which zero-field splitting is resistant to structural variation†
Abstract
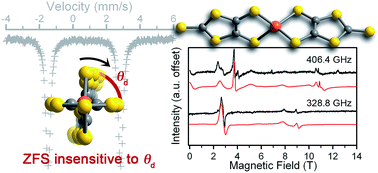
The relationship between electronic structure and zero-field splitting dictates key design parameters for magnetic molecules. In particular, to enable the directed synthesis of new electronic spin based qubits, developing complexes where zero-field splitting energies are invariant to structural changes is a critical challenge. Toward those ends, we report three salts of a new compound, a four-coordinate iron(II) complex [Fe(C3S5)2]2− ([(18-crown-6)K]+ (1), Ph4P+ (2), Bu4N+ (3)) with a continuous structural variation in a single parameter, the dihedral angle (θd) between the two C3S52− ligands, as a function of counterion (θd = 89.98(4)° for 1 to 72.41(2)° for 3). Electron paramagnetic resonance data for 1–3 reveal zero-field splitting parameters that are unusually robust to the structural variation. Mössbauer spectroscopic measurements indicate that the structural variation in θd primarily affects the highest-energy 3d-orbitals (dxz and dyz) of the iron(II) ion. These orbitals have the smallest impact on the zero-field splitting parameters, thus the distortion has a minor effect on D and E. These results represent the first part of a directed effort to understand how spin state energies may be fortified against structural distortions for future applications of qubits in non-crystalline environments.

 Please wait while we load your content...
Please wait while we load your content...