A bio-inspired synthesis of oxindoles by catalytic aerobic dual C–H functionalization of phenols†
Abstract
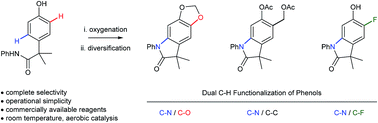
Nitrogen-containing heterocycles are fundamentally important to the function of pharmaceuticals, agrochemicals and materials. Herein, we report a bio-inspired approach to the synthesis of oxindoles, which couples the energetic requirements of dehydrogenative C–N bond formation to the reduction of molecular oxygen (O2). Our method is inspired by the biosynthesis of melanin pigments (melanogenesis), but diverges from the biosynthetic polymerization. Mechanistic analysis reveals the involvement of CuII-semiquinone radical intermediates, which enable dehydrogenative carbon–heteroatom bond formation that avoids a catechol/quinone redox couple. This mitagates the deleterious polarity reversal that results from phenolic dearomatization, and enables a high-yielding phenolic C–H functionalization under catalytic aerobic conditions. Our work highlights the broad synthetic utility and efficiency of forming C–N bonds via a catalytic aerobic dearomatization of phenols, which is currently an underdeveloped transformation.


 Please wait while we load your content...
Please wait while we load your content...