Redox-configurable ambidextrous catalysis: structural and mechanistic insight†
Abstract
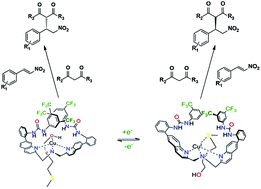
A ligand capable of adopting two pseudo-enantiomeric helically chiral states when bound to copper has been applied as an asymmetric catalyst in the Michael addition of malonate substrates to nitrostyrenes. The absolute configuration of the helically chiral ligand is inverted upon oxidation/reduction of the copper center. In this way, the handedness of the Michael addition product (R/S) can be selected based on the handedness of the catalyst (Λ/Δ). Exciton coupled circular dichroism (ECCD) was used to identify which of the two pseudo-enantiomeric forms the catalyst adopted after reduction/oxidation, with additional support from X-ray crystallographic data. The synthesis of the ligand was achieved in five steps with an overall 61% yield. Enantiomeric excesses of the Michael addition products of up to 72% (S) and 70% (R) were obtained in acetonitrile. The ability to choose the handedness of the product based on the chiral state of the catalyst has been demonstrated with several different solvents, bases, nitrostyrene/malonate substrates, and prochiral malonate substrates. A combination of molecular modelling, crystal structure and kinetic data suggest that one urea moiety of the catalyst ligand likely binds the nitrostyrene substrate while blocking the Re face of the nitrostyrene in the transition state.

 Please wait while we load your content...
Please wait while we load your content...