2,2′-Bipyridyl formation from 2-arylpyridines through bimetallic diyttrium intermediate†
Abstract
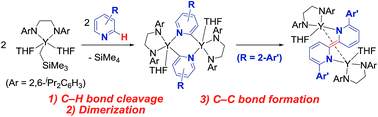
An alkylyttrium complex supported by an N,N′-bis(2,6-diisopropylphenyl)ethylenediamido ligand, (ArNCH2CH2NAr)Y(CH2SiMe3)(THF)2 (1, Ar = 2,6-iPr2C6H3), activated an ortho-phenyl C–H bond of 2-phenylpyridine (2a) to form a (2-pyridylphenyl)yttrium complex (3a) containing a five-membered metallacycle. Subsequently, a unique C(sp2)–C(sp2) coupling of 2-phenylpyridine proceeded through a bimetallic yttrium intermediate, derived from an intramolecular shift of the yttrium center to an ortho-position of the pyridine ring in 3a, to yield a bimetallic yttrium complex (4a) bridged by two-electron reduced 6,6′-diphenyl-2,2′-bipyridyl. Aryl substituents at the ortho-position of the pyridine ring were key in order to destabilize the μ,κ2-(C,N)-pyridyldiyttrium intermediate prior to the C(sp2)–C(sp2) bond formation.

 Please wait while we load your content...
Please wait while we load your content...