Asymmetric C–H functionalization of cyclopropanes using an isoleucine-NH2 bidentate directing group†
Abstract
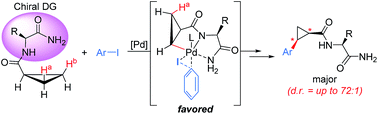
The systematic investigation of substrate-bound α-amino acid auxiliaries has resulted in catalytic asymmetric C–H functionalization of cyclopropanes enabled by amino acid amides as chiral bidentate directing groups. The use of an Ile-NH2 auxiliary embedded in the substrate provided excellent levels of asymmetric induction (diastereomeric ratio of up to 72 : 1) in the Pd(II)-catalyzed β-methylene C(sp3)–H bond activation of cyclopropanes and cross-coupling with aryl iodides.

 Please wait while we load your content...
Please wait while we load your content...