A supramolecular strategy for tuning the energy level of naphthalenediimide: Promoted formation of radical anions with extraordinary stability†
Abstract
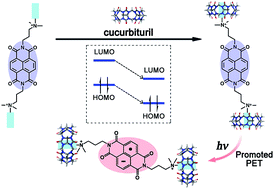
We report a supramolecular strategy to promote and stabilize the formation of naphthalenediimide (NDI) radical anions. The LUMO and HOMO energy of NDI are lowered significantly by introducing cucurbit[7]uril (CB[7]) to each side of a designed NDI molecule through supramolecular complexation. This promotes efficiently the photo-induced electron transfer process between NDI and bromide anions in aqueous solution. The resulting NDI supramolecular radical anions are of outstanding stability. They are even stable in aqueous solution at higher temperatures of 40 °C and 60 °C. It is anticipated that this supramolecular strategy may provide a facile method for stabilizing radicals towards the development of novel materials with spin-based properties and optical properties in the visible and near-infrared regions.

 Please wait while we load your content...
Please wait while we load your content...