Is a polymer semiconductor having a “perfect” regular structure desirable for organic thin film transistors?†
Abstract
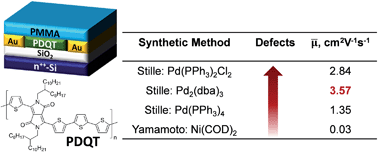
This study utilized high temperature NMR and matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF) mass spectrometry to reveal that appreciable amounts of structural defects are present in the diketopyrrolopyrrole (DPP)–quaterthiophene copolymers (PDQT) synthesized by the Stille coupling polymerization with Pd(PPh3)2Cl2, Pd2(dba)3/P(o-tol)3, and Pd(PPh3)4 catalyst systems. It was proposed that these structural defects were produced via homocoupling side reactions of the C–Br bonds and the organostannane species. Model Stille coupling reactions further substantiated that the amount of structural defects are catalyst-dependent following the order of Pd(PPh3)2Cl2 > Pd2(dba)3/P(o-tol)3 > Pd(PPh3)4. To verify the structural assignments, “perfect” structurally regular PDQT polymers were prepared using Yamamoto coupling polymerization. When compared to the structurally regular polymers, the polymers containing defects exhibited notable redshifts in their absorption spectra. Surprisingly, the “perfect” structurally regular polymers showed poor molecular ordering in thin films and very low charge transport performance as channel semiconductors in organic thin film transistors (OTFTs). On the contrary, all the “defected” polymers exhibited much improved molecular ordering and significantly higher charge carrier mobility.

 Please wait while we load your content...
Please wait while we load your content...