Biosynthesis of trioxacarcin revealing a different starter unit and complex tailoring steps for type II polyketide synthase†
Abstract
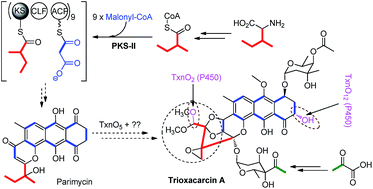
Trioxacarcins (TXNs) are highly oxygenated, polycyclic aromatic natural products with remarkable biological activity and structural complexity. Evidence from 13C-labelled precursor feeding studies demonstrated that the scaffold was biosynthesized from one unit of L-isoleucine and nine units of malonyl-CoA, which suggested a different starter unit in the biosynthesis. Genetic analysis of the biosynthetic gene cluster revealed 56 genes encoding a type II polyketide synthase (PKS), combined with a large amount of tailoring enzymes. Inactivation of seven post-PKS modification enzymes resulted in the production of a series of new TXN analogues, intermediates, and shunt products, most of which show high anti-cancer activity. Structural elucidation of these new compounds not only helps us to propose the biosynthetic pathway, featuring a type II PKS using a novel starter unit, but also set the stage for further characterization of the enzymatic reactions and combinatorial biosynthesis.

 Please wait while we load your content...
Please wait while we load your content...