Exploiting parameter space in MOFs: a 20-fold enhancement of phosphate-ester hydrolysis with UiO-66-NH2†
Abstract
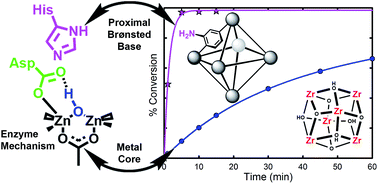
The hydrolysis of nerve agents is of primary concern due to the severe toxicity of these agents. Using a MOF-based catalyst (UiO-66), we have previously demonstrated that the hydrolysis can occur with relatively fast half-lives of 50 minutes. However, these rates are still prohibitively slow to be efficiently utilized for some practical applications (e.g., decontamination wipes used to clean exposed clothing/skin/vehicles). We thus turned our attention to derivatives of UiO-66 in order to probe the importance of functional groups on the hydrolysis rate. Three UiO-66 derivatives were explored; UiO-66-NO2 and UiO-66-(OH)2 showed little to no change in hydrolysis rate. However, UiO-66-NH2 showed a 20 fold increase in hydrolysis rate over the parent UiO-66 MOF. Half-lives of 1 minute were observed with this MOF. In order to probe the role of the amino moiety, we turned our attention to UiO-67, UiO-67-NMe2 and UiO-67-NH2. In these MOFs, the amino moiety is in close proximity to the zirconium node. We observed that UiO-67-NH2 is a faster catalyst than UiO-67 and UiO-67-NMe2. We conclude that the role of the amino moiety is to act as a proton-transfer agent during the catalytic cycle and not to hydrogen bond or to form a phosphorane intermediate.

 Please wait while we load your content...
Please wait while we load your content...