Facile synthesis of oligoyne amphiphiles and their rotaxanes†
Abstract
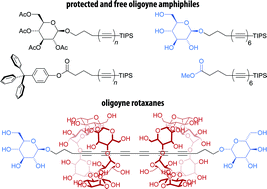
Carbon-rich organic compounds containing a series of conjugated triple bonds (oligoynes) are relevant synthetic targets, but an improved access to oligoynes bearing functional groups would be desirable. Here, we report the straightforward synthesis of two series of oligoyne amphiphiles with glycoside or carboxylate polar head groups, investigate their self-assembly behavior in aqueous media, and their use as precursors for the formation of oligoyne rotaxanes with cyclodextrin hosts. To this end, we employed mono-, di-, or triacetylenic building blocks that gave access to the corresponding zinc acetylides in situ and allowed for the efficient elongation of the oligoyne segment in few synthetic steps via a Negishi coupling protocol. Moreover, we show that the obtained oligoyne derivatives can be deprotected to yield the corresponding amphiphiles. Depending on their head groups, the supramolecular self-assembly of these amphiphiles gave rise to different types of carbon-rich colloidal aggregates in aqueous media. Furthermore, their amphiphilicity was exploited for the preparation of novel oligoyne cyclodextrin rotaxanes using simple host–guest chemistry in water.

 Please wait while we load your content...
Please wait while we load your content...