The effect of host structure on the selectivity and mechanism of supramolecular catalysis of Prins cyclizations†
Abstract
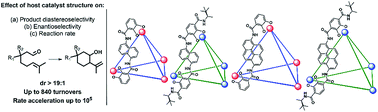
The effect of host structure on the selectivity and mechanism of intramolecular Prins reactions is evaluated using K12Ga4L6 tetrahedral catalysts. The host structure was varied by modifying the structure of the chelating moieties and the size of the aromatic spacers. While variation in chelator substituents was generally observed to affect changes in rate but not selectivity, changing the host spacer afforded differences in efficiency and product diastereoselectivity. An extremely high number of turnovers (up to 840) was observed. Maximum rate accelerations were measured to be on the order of 105, which numbers among the largest magnitudes of transition state stabilization measured with a synthetic host-catalyst. Host/guest size effects were observed to play an important role in host-mediated enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...