Suzuki–Miyaura coupling of arylboronic acids to gold(iii)†
Abstract
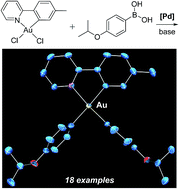
Gold(III) is prominent in catalysis, but its organometallic chemistry continues to be restricted by synthesis. Metal–carbon bond formation often relies on organometallic complexes of electropositive elements, including lithium and magnesium. The redox potential of gold(III) interferes with reactions of these classic reagents. Resort to toxic metals is common, including reagents based on mercury and thallium. We report that the palladium-catalyzed Suzuki–Miyaura coupling of arylboronic acids extends to cyclometalated gold(III) chlorides. Both monoarylation and diarylation are achieved. We propose a mechanism where oxidative addition to palladium with rearrangement at gold(III) fixes the stereochemistry of monoarylated intermediates. Singly arylated species form as thermodynamic isomers. These entities then go on to form diarylated complexes. Reactions proceed at room temperature, and the products are stable to air, moisture, and chromatography.

 Please wait while we load your content...
Please wait while we load your content...