pH-dependent binding of guests in the cavity of a polyhedral coordination cage: reversible uptake and release of drug molecules†
Abstract
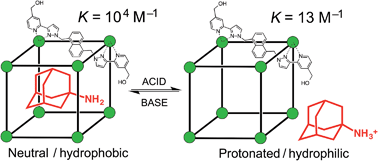
A range of organic molecules with acidic or basic groups exhibit strong pH-dependent binding inside the cavity of a polyhedral coordination cage. Guest binding in aqueous solution is dominated by a hydrophobic contribution which is compensated by stronger solvation when the guests become cationic (by protonation) or anionic (by deprotonation). The Parkinson's drug 1-amino-adamantane (‘amantadine’) binds with an association constant of 104 M−1 in the neutral form (pH greater than 11), but the stability of the complex is reduced by three orders of magnitude when the guest is protonated at lower pH. Monitoring the uptake of the guests into the cage cavity was facilitated by the large upfield shift for the 1H NMR signals of bound guests due to the paramagnetism of the host. Although the association constants are generally lower, guests of biological significance such as aspirin and nicotine show similar behaviour, with a substantial difference between neutral (strongly binding) and charged (weakly binding) forms, irrespective of the sign of the charged species. pH-dependent binding was observed for a range of guests with different functional groups (primary and tertiary amines, pyridine, imidazole and carboxylic acids), so that the pH-swing can be tuned anywhere in the range of 3.5–11. The structure of the adamantane-1-carboxylic acid complex was determined by X-ray crystallography: the oxygen atoms of the guest form CH⋯O hydrogen bonds with one of two equivalent pockets on the internal surface of the host. Reversible uptake and release of guests as a function of pH offers interesting possibilities in any application where controlled release of a molecule following an external stimulus is required.
- This article is part of the themed collection: Global challenges: Health & Food

 Please wait while we load your content...
Please wait while we load your content...