A mild and fast photocatalytic trifluoromethylation of thiols in batch and continuous-flow†
Abstract
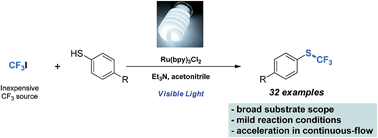
S–CF3 bonds are important structural motifs in various pharmaceutical and agrochemical compounds. However, their preparation remains a major challenge in synthetic organic chemistry. Here, we report the development of a mild and fast photocatalytic trifluoromethylation of thiols. The combination of commercially available Ru(bpy)3Cl2, visible light and inexpensive CF3I gas proved to be an efficient method for the direct trifluoromethylation of thiols. The protocol is demonstrated on a wide range of aromatic, hetero-aromatic and aliphatic substrates in both batch and continuous microflow (32 examples, 52–98% yield). Process intensification through continuous microflow application resulted in a 15-fold increase in production rate (0.25 mmol min−1) due to improved gas–liquid mass transfer, enhanced irradiation as well as convenient handling of the gaseous CF3 source. Furthermore, the efficiency of the flow process allowed to reduce the amount of CF3I (1.1 equivalent) to reach full conversion.

 Please wait while we load your content...
Please wait while we load your content...