Reductive silylation of a uranyl dibenzoylmethanate complex: an example of controlled uranyl oxo ligand cleavage†
Abstract
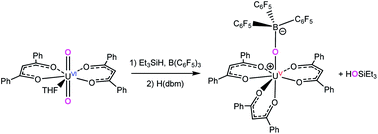
Reaction of UO2(dbm)2(THF) (dbm = OC(Ph)CHC(Ph)O) with 1 equiv. of R3SiH (R = Ph, Et), in the presence of B(C6F5)3, results in the formation of U(OB{C6F5}3)(OSiR3)(dbm)2(THF) (R = Ph, 1; Et, 2), which were isolated as red-orange crystalline solids in good yields. Interestingly, the addition of 1 equiv. of H(dbm) to 2 results in protonation of the –OSiEt3 ligand and formation of U(OB{C6F5}3)(dbm)3 (4) in 33% yield, along with formation of HOSiEt3. Furthermore, addition of HOSiEt3 and 1 equiv. of THF to 4, results in the formation 2, revealing that this process is reversible. The two-step conversion of UO2(dbm)2(THF) to 4 represents a rare example of controlled uranyl oxo ligand cleavage at ambient temperature and pressure. Comparison of diffraction and density functional theory data for 4 suggests the presence of the inverse trans influence, with a very shallow potential energy well for distortion along the trans U–O bond.

 Please wait while we load your content...
Please wait while we load your content...