Electron-accepting phthalocyanine–pyrene conjugates: towards liquid phase exfoliation of graphite and photoactive nanohybrid formation with graphene†
Abstract
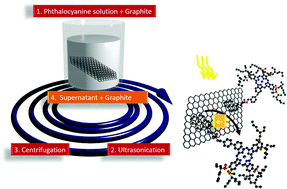
Herein, we describe the synthesis of a zinc(II) alkylsulfonylphthalocyanine–pyrene conjugate, its assembly with highly exfoliated graphite, and the investigation of the photophysical properties of the resulting nanohybrid. The presence of the pyrene unit in the conjugate is decisive in terms of non-covalently immobilizing the electron accepting phthalocyanines onto the basal plane of highly exfoliated graphite. As a matter of fact, strong interactions dominate the electronic properties of the nanohybrid in both the ground and excited states. For example, femtosecond pump probe experiments assist in corroborating an ultrafast charge separation, that is, the generation of the one-electron reduced radical anion of the phthalocyanine and one-electron oxidized graphene after irradiation at 387 nm, followed by slow charge recombination.

 Please wait while we load your content...
Please wait while we load your content...