Rh(iii)-catalyzed C–H functionalization/aromatization cascade with 1,3-dienes: a redox-neutral and regioselective access to isoquinolines†
Abstract
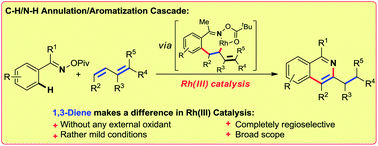
An efficient rhodium(III)-catalyzed redox-neutral C–H activation/cyclization/isomerization strategy to prepare isoquinolines with complete regioselectivity from aromatic oxime esters and diverse 1,3-dienes is described. The advantages of this process are: (1) no need for an external oxidant; (2) simple and convenient reaction conditions; (3) complete regioselectivity; (4) broad scope of substrates.

 Please wait while we load your content...
Please wait while we load your content...