The kinetics of carbonyl radical ring closures†
Abstract
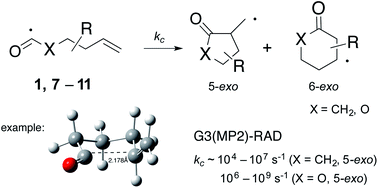
Intramolecular homolytic addition reactions of acyl and oxyacyl radicals 1, 7–11 have been investigated by computational techniques. Acyl radical (X = CH2) cyclizations appear to proceed through Beckwith–Houk transition states that adopt pseudo-chair like structures, while the oxyacyl radicals (X = O) appear to behave more like sp2-hybridized radicals in which the 6-endo transition states are predicted to adopt boat-like structures. G3(MP2)-RAD calculations provide activation energies (Ea) that lie in the range: 24–37 kJ mol−1 (acyl, 5-exo); 17–29 kJ mol−1 (oxyacyl, 5-exo); 32–41 kJ mol−1 (acyl, 6-endo); and 40–50 kJ mol−1 (oxyacyl, 6-endo), with associated log(A/s−1) values in the expected range: 10.5–12.5. These data provide rate constants for 5-exo cyclization (kc) of ∼104 to 107 s−1 for the acyl radicals, and ∼106 to 109 s−1 for the oxyacyl radicals (296 K) and are in excellent agreement with the few available kinetic data. This study provides valuable new information for practitioners wishing to employ this chemistry in synthesis.

 Please wait while we load your content...
Please wait while we load your content...