Spin crossover iron(ii) complexes as PARACEST MRI thermometers†
Abstract
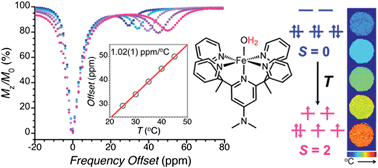
We demonstrate the potential utility of spin crossover iron(II) complexes as temperature-responsive paramagnetic chemical exchange saturation transfer (PARACEST) contrast agents in magnetic resonance imaging (MRI) thermometry. This approach is illustrated in the two molecular complexes [Fe(3-bpp)2]2+ (3-bpp = 2,6-di(pyrazol-3-yl)pyridine) and [(Me2NPY5Me2)Fe(H2O)]2+ (Me2NPY5Me2 = 4-dimethylamino-2,6-bis(1,1-bis(2-pyridyl)ethyl)pyridine). Variable-temperature magnetic susceptibility data collected for aqueous solutions of these complexes reveal that they exhibit spin crossover behaviour in H2O over the temperature range 20–60 °C. Selective presaturation of pyrazolyl and coordinated water protons in these complexes, respectively, leads to a significant decrease in the NMR signal intensity of bulk water protons through CEST. The corresponding Z-spectra reveal a strong linear temperature dependence of chemical shift of those protons, 0.23(1) ppm °C−1 and 1.02(1) ppm °C−1, respectively, arising from thermal conversion between low-spin S = 0 and high-spin S = 2 iron(II), representing 23- and 100-fold higher sensitivity than that afforded by conventional proton resonance frequency shift thermometry. Finally, temperature maps generated for an aqueous solution containing [(Me2NPY5Me2)Fe(H2O)]2+ show excellent agreement with independently measured temperatures of the solution.

 Please wait while we load your content...
Please wait while we load your content...