Regioselective alkyl transfer from phosphonium ylides to functionalized polyfluoroarenes†
Abstract
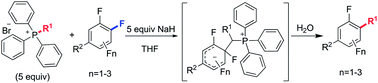
We report an unprecedented alkyl transfer from phosphonium ylides to polyfluoroarenes in a highly regioselective manner. This reaction allows the introduction of a variety of alkyl groups to the para position of functionalized polyfluoroarenes under mild conditions. The process is found to be compatible with several electrophilic functional groups such as carboxyl, ester, amide and cyano groups. NMR spectroscopy studies and deuterated labeling experiments reveal that the reaction proceeds via an SNAr mechanism and the proton that is transferred to the α-carbon of the alkyl group during the last C–P bond breaking step is from water.

 Please wait while we load your content...
Please wait while we load your content...