Catalytic asymmetric direct α-alkylation of amino esters by aldehydes via imine activation†
Abstract
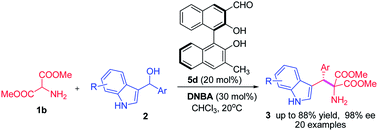
Two types of BINOL-related chiral aldehydes were used as organocatalysts for the direct α-functionalization of N-unprotected amino esters. The first chiral aldehyde catalysed α-alkylation of 2-aminomalonates with 3-indolylmethanols via imine activation was reported. Various tryptophan derivatives were produced in good yields and with high enantioselectivities. A reasonable mechanism was proposed and the core intermediates were identified by high resolution mass spectroscopy (HRMS).

 Please wait while we load your content...
Please wait while we load your content...