Abstract
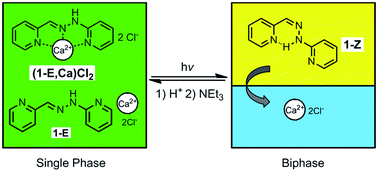
Irradiation of a 3 : 2 acetonitrile–water solution of the bis-pyridyl hydrazone 1 and calcium chloride causes a photo-induced phase separation, due to an increase in free calcium cations as a consequence of the photo-conversion of 1 from its E form to the Z configuration. Complexation studies confirmed that the former binds calcium more strongly that the latter. Acid-catalyzed back-conversion from 1-Z to 1-E, followed by basification, leads to the regeneration of the initial single phase. The process thus involves a photo-thermal cycle with reversible interconversion between monophasic and biphasic states. In the presence of a dynamic library of imines generated from hydrophilic and hydrophobic aldehyde and amine components, a coupling to the phase interconversion processes is achieved, leading to a dynamic redistribution of the imine constituents, whereby amplification of the most lipophilic and the most hydrophilic imine constituents, in the organic and the aqueous phase, respectively, is achieved by component selection. The system thus undergoes an adaptation by the up-regulation of the fittest constituent for its specific phase. The process is reversible, regenerating the initial distribution of constituents upon phase reunification. It also represents the coupling of a dynamic covalent library to out-of-equilibrium conditions resulting from the photo-generation of a kinetically trapped entity.

 Please wait while we load your content...
Please wait while we load your content...