Facile Si–H bond activation and hydrosilylation catalysis mediated by a nickel–borane complex†
Abstract
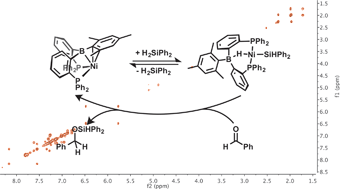
Metal–borane complexes are emerging as promising systems for study in the context of bifunctional catalysis. Herein we describe diphosphineborane nickel complexes that activate Si–H bonds and catalyze the hydrosilylation of aldehydes. Treatment of [MesDPBPh]Ni (1) ([MesDPBPh] = MesB(o-Ph2PC6H4)2) with organosilanes affords the complexes [MesDPBPh](μ-H)NiE (E = SiH2Ph (3), SiHPh2 (4)). Complex 4 is in solution equilibrium with 1 and the thermodynamic and kinetic parameters of their exchange have been characterized by NMR spectroscopy. Complex 1 is a catalyst for the hydrosilylation of a range of para-substituted benzaldehydes. Mechanistic studies on this reaction via multinuclear NMR spectroscopy are consistent with the intermediacy of a borohydrido-Ni-siloxyalkyl species.

 Please wait while we load your content...
Please wait while we load your content...