Effect of the trifluoromethyl group on torquoselectivity in the 4π ring-opening reaction of oxetenes: stereoselective synthesis of tetrasubstituted olefins†
Abstract
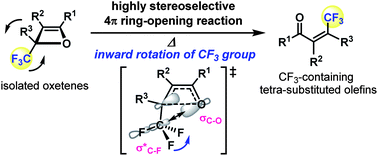
A highly stereoselective synthesis of tetrasubstituted olefins bearing a trifluoromethyl group via the thermal 4π electrocyclic ring-opening reaction of oxetenes, simply prepared by the Pd-catalyzed [2+2] cycloaddition reaction of various alkynes with trifluoropyruvate, is achieved. In this reaction process, the trifluoromethyl group prefers inward rotational torquoselectivity because of the orbital interactions between the breaking C–O σ orbital on the oxetene moiety and the C–F σ* orbital in the transition state.

 Please wait while we load your content...
Please wait while we load your content...