Synthesis, electronic structure and reactivity of bis(imino)pyridine iron carbene complexes: evidence for a carbene radical†
Abstract
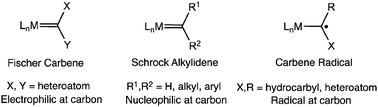
The reactivity of the disubstituted diazoalkane, N2CPh2 with a family of bis(imino)pyridine iron dinitrogen complexes was examined. For the most sterically protected member of the series, (iPrPDI)Fe(N2)2 (iPrPDI = 2,6-(2,6-iPr2C6H3N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)2C5H3N), an S = 1 iron diazoalkane complex was obtained and structurally characterized. Reducing the size of the 2,6-aryl substituents to ethyl or methyl groups resulted in isolation of bis(imino)pyridine iron carbene complexes. Magnetic measurements established S = 1 ground states, demonstrating rare examples of iron carbenes in a weak ligand field. Electronic structure determination using metrical parameters from X-ray diffraction as well as Mössbauer, XAS and computational data established high-spin iron(II) compounds engaged in antiferromagnetic coupling with redox-active bis(imino)pyridine and carbene radicals.
CMe)2C5H3N), an S = 1 iron diazoalkane complex was obtained and structurally characterized. Reducing the size of the 2,6-aryl substituents to ethyl or methyl groups resulted in isolation of bis(imino)pyridine iron carbene complexes. Magnetic measurements established S = 1 ground states, demonstrating rare examples of iron carbenes in a weak ligand field. Electronic structure determination using metrical parameters from X-ray diffraction as well as Mössbauer, XAS and computational data established high-spin iron(II) compounds engaged in antiferromagnetic coupling with redox-active bis(imino)pyridine and carbene radicals.

 Please wait while we load your content...
Please wait while we load your content...