Isothiourea-mediated asymmetric Michael-lactonisation of trifluoromethylenones: a synthetic and mechanistic study†
Abstract
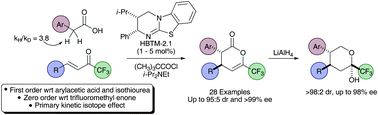
HBTM-2.1 promotes the catalytic asymmetric intermolecular Michael-lactonisation of arylacetic acids and trifluoromethylenones in the presence of

 Please wait while we load your content...
Please wait while we load your content...