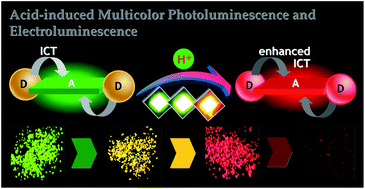
A donor–acceptor–donor (D–A–D) molecule di(triphenylamine)-thiazolothiazole 1 has been employed in which the central Lewis-basic nitrogen atoms prompt it to be responsive to strong acids in both solution and solid states. As a result, the electron-accepting strength and intramolecular charge transfer (ICT) character can be controllably enhanced which make the emission band of 1 sequentially red-shift. The green luminescent solids (524 nm) can be smoothly transferred into yellow (576 nm), red (640 nm), and near infrared (739 nm) emissive states with a huge Δλem of 215 nm after being evaporated by HCl, TFA, and Lewis acid BBr3, respectively. Taking advantage of the smart sensitivity of 1 to acids, we prepared thermal-evaporated OLEDs based on the neutral and protonated species for the first time and achieved multicolor electroluminescence (EL) with very high brightness and moderate current efficiency. Thus, multicolor photoluminescence and thermally evaporated electroluminescence have been firstly realized based on a single organic chromophore, to the best of our knowledge.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...