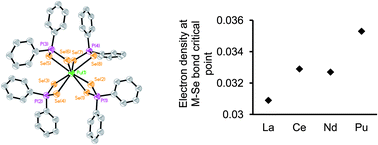
Understanding the bonding trends within, and the differences between, the 4f and 5f element series with soft donor atom ligands will aid elucidation of the fundamental origins of actinide (An) versus lanthanide (Ln) selectivity that is integral to many advanced nuclear fuel cycle separation concepts. One of the principal obstacles to acquiring such knowledge is the dearth of well characterized transuranic molecules that prevents the necessary comparison of 4f versus 5f coordination chemistry, electronic structure, and bonding. Reported herein is new chemistry of selenium analogues of dithiophosphinate actinide extractants. LnIII and AnIII/IV complexes with the diselenophosphinate [Se2PPh2]− anion have been synthesized, structurally and spectroscopically characterized, and quantum chemical calculations performed on model compounds in which the phenyl rings have been replaced by methyl groups. The complexes [LnIII(Se2PPh2)3(THF)2] (Ln = La (1), Ce (2), Nd (3)), [LaIII(Se2PPh2)3(MeCN)2] (4), [PuIII(Se2PPh2)3(THF)2] (5), [Et4N][MIII(Se2PPh2)4] (M = Ce (6), Pu (7)), and [AnIV(Se2PPh2)4] (An = U (8), Np (9)), represent the first f-element diselenophosphinates. In conjunction with the calculated models, complexes 1–9 were utilized to examine two important factors: firstly, bonding trends/differences between trivalent 4f and 5f cations of near identical ionic radii; secondly, bonding trend differences across the 5f series within the AnIV oxidation state. Analysis of both experimental and computational data supports the conclusion of enhanced covalent bonding contributions in PuIII–Se versus CeIII–Se bonding, while differences between UIV–Se and NpIV–Se bonding is satisfactorily accounted for by changes in the strength of ionic interactions as a result of the increased positive charge density on NpIV compared to UIV ions. These findings improve understanding of soft donor ligand binding to the f-elements, and are of relevance to the design and manipulation of f-element extraction processes.

 Please wait while we load your content...
Please wait while we load your content...