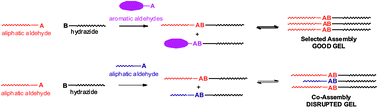
In this paper we report a new, simple gelation system based on an acyl hydrazone building block. These gels can be formed by simple mixing of aldehyde and hydrazide followed by a heat/cool cycle, and constitute a simple type of multi-component gel, in which a new covalent bond is formed. Importantly, we demonstrate two different outcomes when this gelation system is challenged with mixtures of different aldehydes – in some cases, only one of the aldehydes will react with the hydrazide to preferentially form the effective gelator and assemble into organised gels, while in other cases, both aldehydes will react with the hydrazide to yield co-assembled systems. Detailed insight into these assembly processes is obtained through NMR and differential scanning calorimetry. Understanding self-organising systems is a vital step towards the self-assembly of multi-functional advanced materials from complex systems, and also indicates how self-assembly processes, when coupled with chemical reactivity, allow order to spontaneously rise from chaotic mixtures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...