Retracted Article: Homonuclear bond activation using a stable N,N′-diamidocarbene†
Abstract
The
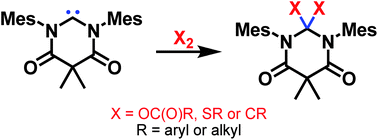
Maintenance work is planned for Wednesday 1st May 2024 from 9:00am to 11:00am (BST).
During this time, the performance of our website may be affected - searches may run slowly and some pages may be temporarily unavailable. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.
* Corresponding authors
a
Department of Chemistry and Biochemistry, The University of Texas at Austin, 1 University Station A1590, Austin, TX, USA
E-mail:
bielawski@cm.utexas.edu
The
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
This article has not yet been cited.
Loading related content
