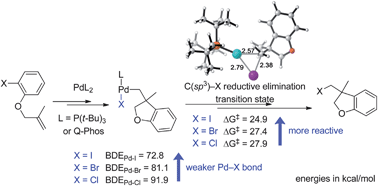
The mechanism of Pd(0)-catalyzed carbohalogenation of alkenes has been investigated with density functional theory. The catalytic cycle involves oxidative addition of the aryl halide, alkene insertion, and a novel C(sp3)–I reductive elimination. The C(sp3)–I reductive elimination leads to a weakly bonded Pd–product complex, which undergoes ligand exchange via a dissociative mechanism to regenerate the catalyst. In the rate-determining reductive elimination step, bromides and chlorides have higher barriers than iodides, because the stronger Pd–Br and Pd–Cl bonds are being cleaved in these transition states. Bulky ligands, such as P(t-Bu)3 and Q-Phos, facilitate the C(sp3)–I reductive elimination by preventing the formation of tetracoordinated intermediates. The mechanism of the competing β-hydrogen elimination pathway was also investigated. For reactions involving a syn-β-hydrogen atom in the alkyl Pd(II) iodide intermediate, β-hydrogen elimination is much more favorable, leading to Heck-type side products. Blocking β-elimination by the choice of substrates is the main reason why this example of carboiodination works.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...