Breathing molecular crystals: halogen- and hydrogen-bonded porous molecular crystals with solvent induced adaptation of the nanosized channels†
Abstract
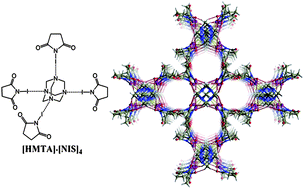
Exceptionally strong (OC–)2N–I⋯N halogen bonding (XB) in a combination with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–C hydrogen bonds (HB) between N-iodosuccinimide (NIS) and hexamethylenetetramine (HMTA) yielded a series of molecular crystals possessing large 1D channels. In each structure, HMTA was tetra-coordinated by four NIS molecules resulting in robust [HMTA]·[NIS]4 complexes where the observed I⋯N distances, ranging from 2.486 to 2.586 Å, were remarkable shorter (from 29.6 to 26.7%) than the sum of the vdW radii of nitrogen and iodine atoms. Multiple C
O⋯H–C hydrogen bonds (HB) between N-iodosuccinimide (NIS) and hexamethylenetetramine (HMTA) yielded a series of molecular crystals possessing large 1D channels. In each structure, HMTA was tetra-coordinated by four NIS molecules resulting in robust [HMTA]·[NIS]4 complexes where the observed I⋯N distances, ranging from 2.486 to 2.586 Å, were remarkable shorter (from 29.6 to 26.7%) than the sum of the vdW radii of nitrogen and iodine atoms. Multiple C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–C HBs interconnected the [HMTA]·[NIS]4 complexes into the structures with flexible “breathing” host-channels. Three different host-channel structures, either oval or cylindrically shaped with volumes of about 20, 28 or 38% (700–1800 Å3) of the unit cell volume, were obtained to match the selected guest. This adaptability was taken further by guest molecule exchange experiments, where CH2Cl2 were exchanged with CCl4 in solution and from gas-to-solid reaction. Both experiments lead to the same single-crystal to single-crystal transformation (P43212, V = 4642 Å3 to P42/nmc, V = 2324 Å3) with halving the unit cell volume.
O⋯H–C HBs interconnected the [HMTA]·[NIS]4 complexes into the structures with flexible “breathing” host-channels. Three different host-channel structures, either oval or cylindrically shaped with volumes of about 20, 28 or 38% (700–1800 Å3) of the unit cell volume, were obtained to match the selected guest. This adaptability was taken further by guest molecule exchange experiments, where CH2Cl2 were exchanged with CCl4 in solution and from gas-to-solid reaction. Both experiments lead to the same single-crystal to single-crystal transformation (P43212, V = 4642 Å3 to P42/nmc, V = 2324 Å3) with halving the unit cell volume.

 Please wait while we load your content...
Please wait while we load your content...