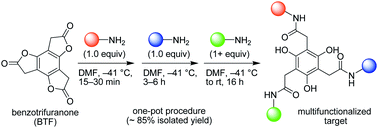
Synthetic access to discrete multifunctionalized molecules remains a challenge but is critical for continued advances in materials science, catalysis, and chemical biology. Here, the aminolysis of electronically coupled lactones is introduced as an approach to achieve molecular multifunctionalization efficiently, under mild conditions, and without protecting groups or by-products. The one-pot sequential aminolysis of benzotrifuranone, a C3h-symmetric trilactone, to produce a trifunctionalized target in ∼85% yield over one day is highlighted.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...