Abstract
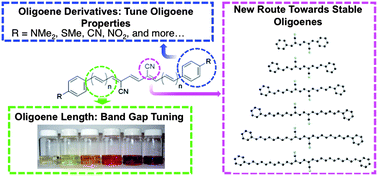
We describe the synthesis and characterization of a new class of cyano-functionalized oligoenes and their derivatives. We have made the vinylogous series of α,ω-diphenyl-μ,ν-dicyano-oligoenes (DPDCn) comprised of each odd-numbered member from 3 to 13 linear conjugated

 Please wait while we load your content...
Please wait while we load your content...