Spectacular luminescent behaviour of tandem terpyridyl platinum(ii) acetylide complexes attributed to solvent effect on ordering of excited states, “ion-pair” formation and molecular conformations†
Abstract
A series of oligomeric tandem terpyridyl platinum(II) complexes, namely [(tBu3tpy)Pt(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ctpy)PtCl](OTf)2 (3), [(tBu3tpy)Pt(C
Ctpy)PtCl](OTf)2 (3), [(tBu3tpy)Pt(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ctpy)PtC
Ctpy)PtC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu](OTf)2 (4), [(tBu3tpy)Pt(C
CtBu](OTf)2 (4), [(tBu3tpy)Pt(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ctpy)PtC
Ctpy)PtC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ctpy](OTf)2 (5), and [(tBu3tpy)Pt(C
Ctpy](OTf)2 (5), and [(tBu3tpy)Pt(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ctpy)Pt(C
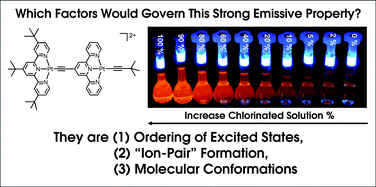
Ctpy)Pt(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ctpy)PtCl](OTf)3 (6), were prepared and their spectroscopic properties and self-aggregating behaviour were examined. In particular, complex 4 exhibits unusually higher emission quantum yield in CH2Cl2 (ϕ = 0.43) than that in CH3CN (ϕ < 0.1), which is attributed to the formation of a “contact ion pair” in chlorinated
Ctpy)PtCl](OTf)3 (6), were prepared and their spectroscopic properties and self-aggregating behaviour were examined. In particular, complex 4 exhibits unusually higher emission quantum yield in CH2Cl2 (ϕ = 0.43) than that in CH3CN (ϕ < 0.1), which is attributed to the formation of a “contact ion pair” in chlorinated

 Please wait while we load your content...
Please wait while we load your content...