Kinetics and computational fluid dynamics study for Fischer–Tropsch synthesis in microchannel and fixed-bed reactors†
Abstract
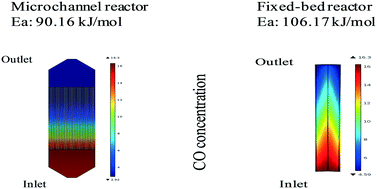
The effect of operating conditions on the hydrocarbon yield distribution during Fischer–Tropsch synthesis (FTS) in a microchannel reactor was studied. A power law-based kinetic model was developed for the first time in microchannel and fixed bed reactors for FTS reactions. The activation energy calculated was 90.16 kJ mol−1 and 106.17 kJ mol−1 in the microchannel reactor and fixed bed reactor, respectively. In a single pass run, the CO conversion obtained in the microchannel reactor was more than 92%, while it was 70% in the fixed bed reactor over the same catalyst. The concentration and temperature profile are predicted in both fixed bed and microchannel reactors. As expected, there was no axial concentration gradient observed in the microchannel reactor. Under adiabatic conditions, kinetic and thermodynamic study simulations showed an increase in reactor temperature from 598 K to 639 K in the microchannel reactor and 598 K to 607 K in the fixed bed reactor. The heat produced per unit volume of the microchannel reactor is higher due to a higher rate of the reaction compared to that in the fixed bed reactor.


 Please wait while we load your content...
Please wait while we load your content...