Development of semi-continuous chemo-enzymatic terpene epoxidation: combination of anthraquinone autooxidation and the lipase-mediated epoxidation process†
Abstract
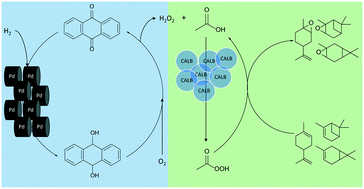
Lipase has been used for epoxidizing olefins such as monoterpenes for more than two decades. This epoxidation is accomplished by adding hydrogen peroxide (H2O2) to a carboxylic acid in the presence of a lipase such as Candida antartica lipase B (CALB) to produce percarboxylic acid, which then epoxidizes monoterpenes according to the Prilezhaev mechanism. One drawback of this process is the need for continuous addition of hydrogen peroxide to maintain maximum productivity. To overcome this hurdle, the industrial anthraquinone autooxidation process for hydrogen peroxide production was scaled down and coupled with lipase-mediated epoxidation in a semi-continuous manner. Palladium on alumina pellets (5% loading) was used as the catalyst for obtaining high yields of high-concentration hydrogen peroxide (50% weight by volume), followed by epoxidation of 3-carene, (+) limonene, and α-pinene. A total reaction time of 5 h was used for hydrogen peroxide production and 2–3 h for the epoxidation reactions. Pure 3-carene epoxide and α-pinene epoxide were obtained in isolated yields of 88.8 ± 2.8% and 83.8 ± 2.6%, respectively. Limonene epoxide was obtained as a mixture of mono- and di-epoxides in a ratio of 70% and 30%, respectively, with an isolated yield of 71.5 ± 3.1%.


 Please wait while we load your content...
Please wait while we load your content...